Lithium
The transition to clean energy
Clean electrification means switching the grid to carbon-free sources. And then shifting technologies that previously relied on combustion onto the grid.
Battery technologies that enable this transition is built on lithium. Hence lithium is accelerating the shift to a renewable, sustainable and low-carbon future.
Lithium Elements
Lithium Carbonate
Lithium carbonate is an inorganic compound and appears as a white, odorless powder. It has applications in the processing of metal oxides and as a precursor for compounds used in lithium-ion batteries. It is also useful as a drug in treating mood disorders.
Importance
Lithium is important for decarbonizing two of the largest emitting sectors - transportation and electricity.
Transportation
The future of cars is electric. Lithium is the main element in rechargeable batteries for electric vehicles (EVs) enabling ever-higher performance and range. Beyond passenger vehicles, EV segments expected to grow rapidly includes buses, haulage vehicles and scooters.
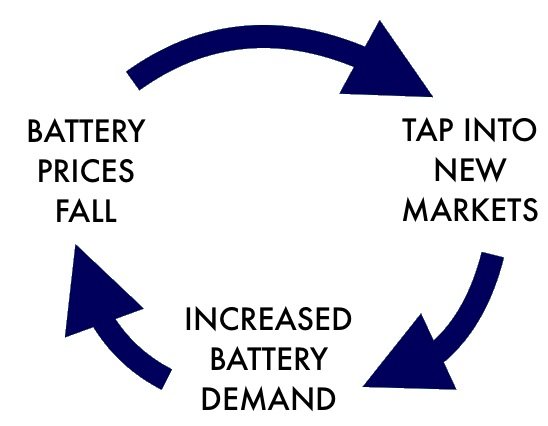
Virtuous Cycle of Energy Storage
Lithium batteries dominate the storage market and have a considerable lead over other technologies in the sector in terms of manufacturing capacity and expertise. Continued scaling will ensure that lithium batteries get cheaper and more powerful. As a result we will find new uses for them. The result is a "virtuouse cycle" that propells the use of lithium batteries further ahead of alternatives.
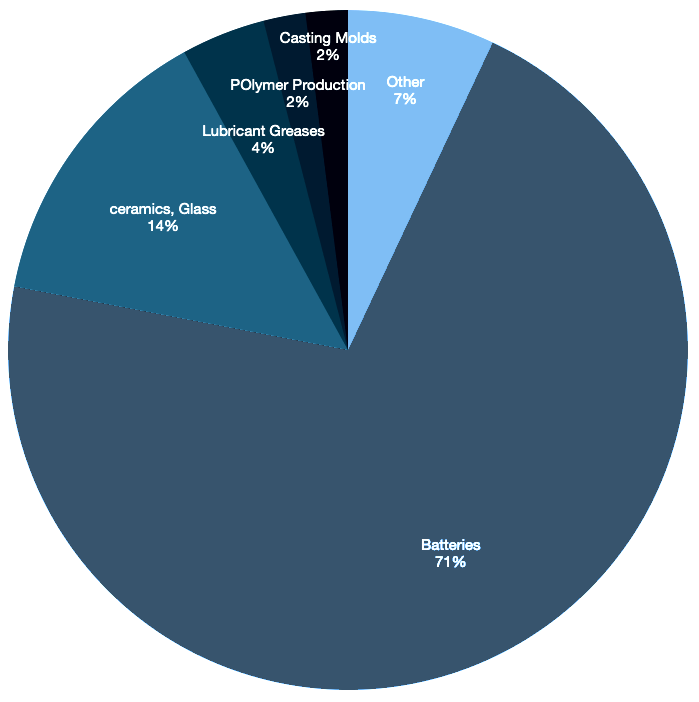
In addition to its use as electrode and electrolyte material in batteries, lithium is used in glass manufacturing, ceramics, pharmaceuticals, and aluminum and magnesium alloys.
Medical
Lithium carbonate is used as a psychiatric medication to treat bipolar disorder.
Batteries
Lithium-ion batteries are used in EVs and are the key to light weight, rechargeable power for laptops, phones and other digital devices.
Glass
Lithium is present in lighting fixtures and LCD screens.
Ceramics
Lithium oxide is used as a flux for processing silica, reducing the melting point and viscosity of the material and for improved thermal expansion.
Metallurgy
Lithium compounds are used as additives to foundry sand for casting iron and as an additive to aluminium smelters.
Lubricating Greases
Lithium is used to manufacture high-temperature, high-load lubricating greases.
Applications
The demand for lithium is tied to the exponential growth in EV production
Driven by government measures, lower vehicle prices, and increasing vehicle model choices from the likes of VW, GM and Ford trying to match Tesla's success, lithium demand is expected to increase to approximately 1.3 million metric tons of LCE (lithium carbonate equivalent) by 2025. This represents a five fold increase from 2020 levels and by 2040 demand is expected to increase 40-fold.
source: ZME Sciences
Electric Vehicle Sales
World Quarterly EV Sales
Lithium Production
Between 2008 and 2018 annual production rose from 25,400 to 85,000 tons of lithium. Supply from mine and brine operations since 2018 has lagged demand growth (other than a dip in 2020 due to covid). New project development is underway, but will take time to fill the supply gap.
Prices
Lithium Carbonate and Lithium Hydroxide
Surging EV demand has resulted in lithium prices skyrocketing by 550% from 2020 to 2021
By the beginning of March 2022, lithium carbonate prices had passed $75,000 per metric ton
Lithium hydroxide prices exceeded $65,000 per metric ton in 2022, compared with a five-year average of $14,500 per metric ton.
Year Price/Ton 1 year % Change
2022. $ 6,100 172%
2021 $ 2,240 433%
2020 $ 420 average
Lithium (Li) is one of the primordial elements produced in Big Bang nucleosynthesis, along with helium and hydrogen.
Lithium is a comparatively rare element and constitutes less than 0.002 percent of Earth's crust. Although widely distributed on Earth it occurs in very low concentrations and not in elemental form due to its high reactivity.
Lithium is commonly found in igneous rocks, with the largest concentrations in granitic pegmatites with spodumene and petalite being the most commercially viable sources. The other major source of lithium is brine deposits.
There are a fairly large number of both lithium mineral and brine deposits but only comparatively few are commercially viable. Most are too small or too low in grade.
Lithium Metal
Lithium is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal, it floats on water. It is also solid at a wide range of temperatures, with one of the lowest melting points of all metals and a high boiling point.
Lithium is highly reactive and is never found in its elemental, metallic form. Excellent heat and electrical conductivity characteristics make it useful for manufacturing pharmaceuticals, glass, lubricants, and most importantly, lithium-ion batteries for electric cars and consumer electronics.
Lithium Hydroxide
Similar to lithium carbonate it is inorganic, and takes the form of white hygroscopic solids. It is usually produced industrially from lithium carbonate in a metathesis reaction with calcium hydroxide and consumed in the production of cathode materials for lithium ion batteries. Frequently used for the production of lubricating greases, particularly those greases required to work under extreme temperatures and loads.
Electricity
Grid scale storage solutions based on lithium ion technology balances loads as dispatchable sources of energy (coal and methane) are phased out and more variable, weather-dependent sources (sun and wind) are used, allowing the grid to remain stable.
Portability
As the lightest metal on the periodic table, and the one most eager to shed its electrons, lithium is the ideal element to make powerful, portable batteries. Lithium batteries retain their charge for longer compared to ni-cd and lead alternatives and are composed of less toxic materials.
Pyrotechnics
Lithium burns bright red and is used as a colorant and oxidizer in fireworks and flares.
Air Purification
Lithium compounds are used in confined spaces (like spacecraft and submarines) for carbon dioxide removal.
Optics
Lithium fluoride may be artificially grown as crystals and used in scientific research for IR and UV applications.
Nuclear
Lithium-6 is a source material for tritium production and as a neutron absorber in nuclear fusion.
Rocket Fuel
Metallic lithium and lithium aluminum hydride are used by the military as rocket propellants.
Construction
Lithium is used in tile adhesives. It is present in cement, used to improve resistance and compression and to accelerate setting.
Demand
Source: Bloomberg, Department of Energy, Science & Resources
Source: AUS Resources and Energy Quarterly
Lithium Sources
Spodumene
By far the largest producer of lithium from spodumene is Australia, which has a large deposit near Perth. The process consists of hard rock mining with the lithium extracted from spodumene via a chemical roasting process. A concentrate with at least 6% Li2O (approximately 75% spodumene) is usually required for this to be viable.
Clay
A chemical process is used to leach lithium from clay ore. Clay minerals are mixed with an aqueous solution of acid (sulphuric acid or hydrochloric acid) and then heated under atmospheric pressure to leach out the lithium contained in the clay minerals.
Year. Tons
2018 85K
2020 464K
2021 485K
2022 600K +
2023 800K +
Brine Pools
This is primarily an evaporation process that takes place over years, usually in remote locations. Water is pumped into the earth to create a brine that is captured in storage ponds. Over the course of 18-24 months natural evaporation occurs, and the resulting material is lithium carbonate.
Spent Batteries
Typically less than 5% of lithium batteries are recycled. It's five times more expensive to obtain lithium from spent batteries than by mining lithium. The process is complex and can be dangerous due to electrical, thermal and chemical interations. It may only be viable if other useful metals are simultaneously obtained, such as cobalt, nickel, copper, aluminium and rare earths.
Supply
EV Long Term Sales Projections
Source: AUS Resources and Energy Quarterly
Spodumene
A similar price trend applies to spodumene, the mineral ore from which more than 50% of lithium is produced.
Spodumene is traded via forward contracts and auctions months in advance of delivery between a few large players. The spot price market for spodumene has only recently emerged so it is difficult to track precise prices.
The table illustrates pricing trends since 2020 for spodumene (6% Li2O min)
Global Lithium Reserves
Price hikes in 2015 triggered concern that lithium resources were insufficient to meet demand from the growing battery industry, and there were predictions that the world was running out of lithium.
Continued exploration since 2016 has uncovered new deposits and current estimates of reserves have been increasing along with demand.
Worldwide lithium reserves identified by USGS between 2016 to 2020 rose from 41 million tonnes to more than 80 million tonnes.
The reality is that lithium deposits are relatively common. We already know of many, and with further exploration more will be identified. Based on current trends every continent and most countries will likely have a lithium deposit, either already known or that could be found with exploration.
Australia
50% of the world's lithium produced via hard rock spodumene mining.
Chile, Argentina, Bolivia
Produces 30% of the world's lithium in a region known as the Lithium Triangle in the Atacama Desert.
China
The world's third-largest producer. Dominates refining and battery manufacturing stages of lithium-ion supply chain.
United States
Less than 1% of global production with a single production site in Nevada.